Politicians at all levels have been lied to about the WHO treaty and int'l health regulation amendments--so show them the screenshots. Here are the receipts!
Full copies of the IHRs and all WHO treaty drafts can be found at https://doortofreedom.org/who-resource-page/
I start out with the International Health Regulations (IHR) amendments. We don’t have an official version later than February 2023, so we don’t know exactly what will be voted on next month, but this is what the public has been shown. Everything inside a thin border with white background is verbatim from the proposed IHR Amendments.
The IHR amendments as well as the Pandemic Treaty specify that “potential pandemic pathogens” [both their sequences and actual specimens] are to be shared with the WHO and then shared globally, with the only proviso here being that WHO will not share them with “conflict and violence elements” once notified not to by a state party.
Now we move to screenshots from different versions of the Pandemic Treaty, which has had many names, but has been called the Pandemic Agreement since last October 2023. You can see that while the treaty claims to support unhindered access to information, a few pages later it demands that nations manage “infodemics” and censor misinformation and disinformation. This is typical of drafts of the treaty: it says one thing in one place and then elsewhere maintains the opposite, allowing politicians to claim the treaty asserts it does not grab any sovereignty, when it clearly does exactly that.
Another example of the WHO’s weasel words is the issue of health coverage. The WHO wants everyone to be required to pay for health insurance, and they imply that this will entitile us to a full panoply of healthcare services. But they have nothing in the documents that even begins to address providing expansive healthcare, nor even basic healthcare, for people.
An example of how sovereignty is to be grabbed is the demand for all nations to pass laws to allow the use of EUA (untested) vaccines and drugs, and the demand to pass laws to waive liability for such products.
The fact that the WHO wants to be the purveyor of open source biological warfare agents, also known as “potential pandemic pathogens” is so bizarre and extraordinary. It reflects the fact the diplomats think we are all morons and cannot see through what they are proposing—a mechanism to ensure pandemics can be started at will, by almost anyone who has received their share of these agents or has access to their online genetic sequences.
Below is from the March 7, 2024 Treaty draft. The treaty is in effect 24/7, which means that the required sharing of pathogens is to occur regardless of whether there is a pandemic or potential pandemic. It is not restricted to a response to a public health threat. It is an activity that is to occur year round, and nations are encouraged to find potential pandemic pathogens for which they can be rewarded monetarily. Wide sharing, beyond the WHO’s lab network, by the WHO and the party that found the pathogen is encouraged.

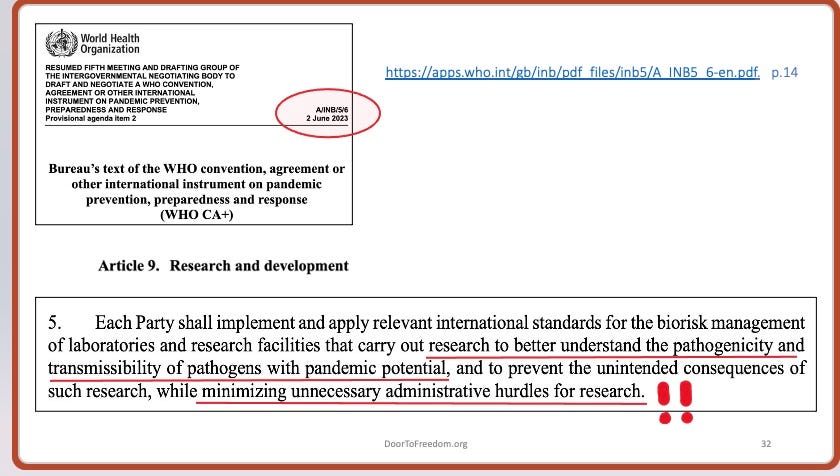
The screenshot below is from a version of the treaty that was issued in June 2023. At that time the draft coyly does not use the terminology “gain of function” but instead defines it, using the coded language “research to better understand the pathogenicity and transmissibility of pathogens with pandemic potential” to describe it. What Article 9, paragraph 5 really says is that if you are performing Gain of Function research, then “minimiz[e] unnecessary administrative hurdles for [that] research.” In other words, loosen the strictures, and thereby enable more lab accidents/spills/leaks to occur.
What does all this mean? First, the WHO lies and misleads, repeatedly. It is not an honest broker and should not be trusted by any nation.
Second, it is attempting to lead us into a pandemic era. One in which the WHO budget will skyrocket to at least ten times its current level, according to the World Bank.
Third, the WHO is breaking international laws and norms to do so. It must be stopped.